Papilloplex High Risk HPV
Human papillomavirus (HPV) infection is the most common sexually transmitted infection worldwide. The link between persistent infection and cervical cancer has led to the emergence of cancer screening programs to better stratify women and their risk of developing cancer. Over 150 types of HPV have been identified, some of which may have major risk factors for cervical cancer. The clinical significance of persistence and virus clearance rates over time on severity and progression, as well as co-infection of high- and low-risk HPV types, have recently been identified as factors for progression of disease. However, current HPV diagnostic tools are limited in their use for the detection, identification and differentiation of multiple HPV genotypes.
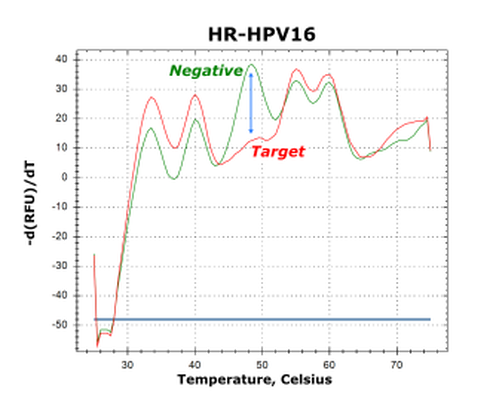
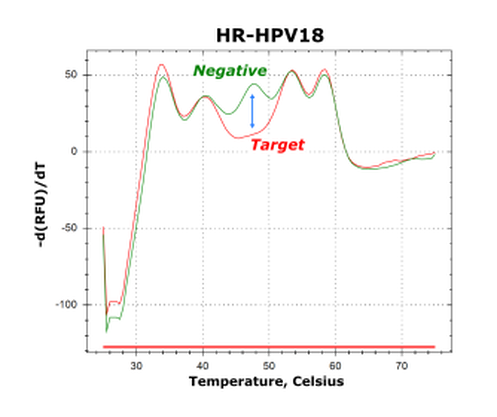
The GeneFirst Papilloplex® High-Risk HPV Test was designed to meet these medical needs by targeting 14 HPV genotypes to better stratify risk and disease progression. This test, based on GeneFirst's proprietary Multiplex Probe Amplification (MPA) technology, helps to better understand the infection of specific HPV genotypes in developing strategies to improve the prevention and management of cervical cancer.
The GeneFirst Papilloplex® High-Risk HPV Test was designed to meet these medical needs by targeting 14 HPV genotypes to better stratify risk and disease progression. This test, based on GeneFirst's proprietary Multiplex Probe Amplification (MPA) technology, helps to better understand the infection of specific HPV genotypes in developing strategies to improve the prevention and management of cervical cancer.
|
Key features and Benefits
|
Technical Specifications
|